Consult with Dr. Laura Geige for Dermal Fillers Now
Risk of Complications
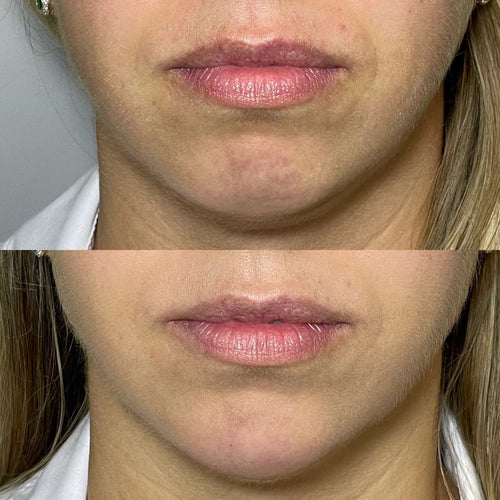
Tear trough filler injections are a popular cosmetic procedure used to alleviate the appearance of dark circles, hollow eyes, and excess skin under the eyes.
However, as with any invasive medical treatment, there are risks associated with tear trough filler injections that patients should be aware of before undergoing the procedure.
Risk of Complications: The most common complications associated with tear trough filler injections include temporary redness, swelling, bruising, and pain at the injection site.
In rare cases, more serious complications can occur, such as infection, bleeding, or scarring. It is essential for patients to choose a qualified and experienced healthcare professional to minimize the risk of complications.
General Risks Associated with Tear Trough Filler Injections:
Allergic Reactions: Some individuals may be allergic to the filler materials used in tear trough injections, which can cause a range of symptoms from mild hives to life-threatening anaphylaxis.
Skin Irritation: The filler materials can cause skin irritation, including redness, swelling, and itching, especially if the patient has sensitive skin or allergies.
Infection: As with any invasive medical treatment, there is a risk of infection with tear trough filler injections. Patients should follow their healthcare professional’s post-procedure instructions carefully to minimize this risk.
Bleeding: Bleeding can occur at the injection site, especially in patients who are taking anticoagulant medications or have bleeding disorders.
Scarring: While rare, scarring can occur if the filler material is not absorbed by the body or if there is an infection.
Asymmetrical Results: Tear trough injections may not produce symmetrical results, and some patients may experience uneven or lopsided outcomes.
Filler Migration: In rare cases, the filler material can migrate from the injection site to other areas of the face, causing unintended consequences such as swelling, bruising, or deformity.
Pain and Sensitivity: Some patients may experience pain or sensitivity in the treated area, especially if the filler material is not absorbed by the body or if there is an allergic reaction.
It is essential for patients to discuss these risks with their healthcare professional before undergoing tear trough filler injections to ensure they understand the potential complications and make an informed decision about their treatment options.
The risk of complications from tear trough fillers is a common concern for individuals considering this cosmetic treatment.
According to various studies and reports, the most common complications reported include bruising, swelling, redness, and swelling at the injection site.
Bruising and swelling are generally temporary and resolve on their own within a few days.
Redness can persist for longer periods, typically up to two weeks, but it is usually mild and manageable with over-the-counter pain medication.
Swelling at the injection site can be more significant and may take several weeks to dissipate.
Less common complications include infection, bleeding, and nerve damage.
Infection can occur if proper sanitation and sterile technique are not followed during the treatment.
Bleeding can happen if the filler is injected too close to a blood vessel.
Nerve damage can result in numbness, tingling, or pain in the area surrounding the injection site.
These complications are often more severe with fillers that contain poly-L-lactic acid (PLLA) or calcium hydroxylapatite.
The risk of complications may also be higher for individuals with certain medical conditions, such as bleeding disorders or nerve damage.
Fillers made from hyaluronic acid are generally considered to have a lower risk of complications compared to PLLA or calcium hydroxylapatite fillers.
However, it’s essential to note that even with the best filler materials and proper technique, complications can still occur.
The key to minimizing risks is to find an experienced and licensed healthcare professional who has performed numerous tear trough injections.
A thorough consultation and medical history review are crucial in determining the best course of treatment and potential risks.
It’s also essential to follow post-treatment instructions carefully, including applying cold compresses to reduce swelling and bruising.
The use of tear trough fillers, such as hyaluronic acid, calcium hydroxylapatite, or poly-L-lactic acid, can potentially cause complications due to various reasons. One possible complication that has been reported in some patients is temporary eyelid swelling or dryness.
This phenomenon occurs when the filler material interacts with the tissues surrounding the tear trough area, causing inflammation and subsequent swelling or dryness of the eyelids. The severity of this complication can vary from mild to moderate, depending on individual factors such as the type of filler used, its concentration, and the patient’s overall health.
Temporary eyelid swelling or dryness is usually a reversible condition that resolves on its own within a few weeks after treatment. However, in some cases, it may take several months for the symptoms to completely subside. In rare instances, the swelling can be more severe and persistent, leading to temporary vision disturbances or discomfort.
Several factors contribute to the risk of temporary eyelid swelling or dryness following tear trough filler injections:
- Overcorrection: Injecting too much filler material into the tear trough area can cause excessive inflammation and swelling, which may lead to temporary eyelid dryness.
- Filler material type and concentration: Different types of filler materials have varying levels of bioabsorbability and reaction rates. Some fillers, like hyaluronic acid, are more prone to causing localized reactions, including swelling or dryness, compared to others, such as calcium hydroxylapatite.
- Patient’s overall health: Patients with pre-existing eye conditions, allergies, or autoimmune disorders may be more susceptible to adverse reactions, including eyelid swelling or dryness.
- Injection technique and site selection: Improper injection techniques or selecting the wrong location for filler placement can increase the risk of complications, including temporary eyelid swelling or dryness.
- Allergic reactions: Although rare, patients may experience allergic reactions to the filler material, which can manifest as itching, redness, or swelling around the injection site, including the eyelids.
The risk of complications from tear trough fillers is generally considered low when performed by an experienced and skilled practitioner. However, it’s essential for patients to follow post-injection instructions carefully, attend scheduled follow-up appointments, and report any adverse reactions or concerns promptly to minimize the likelihood of complications.
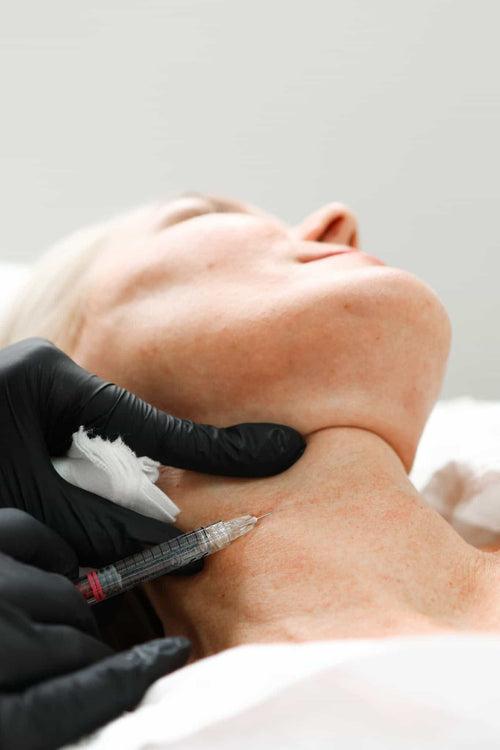
Risk of complications associated with tear trough fillers can be a significant concern for individuals considering this cosmetic treatment.
- In rare cases, more serious complications have been reported, including eye infections, vision loss, or even facial paralysis.
- Eye infections are a potential risk, as bacteria from the filler material can enter the eye and cause infection, potentially leading to vision loss or other complications.
- Vision loss is a rare but possible complication of tear trough fillers, which can occur if the filler material is not properly injected or if there is an adverse reaction.
- Facial paralysis is another rare but serious potential risk of tear trough fillers, as the filler material can cause nerve damage or affect the nerves that control facial muscles, leading to weakness or paralysis of the affected area.
These complications highlight the importance of seeking treatment from a qualified and experienced healthcare professional who uses the latest techniques and materials to minimize the risk of adverse reactions.
A thorough medical history, skin examination, and discussion of potential risks and benefits can help individuals make an informed decision about whether tear trough fillers are right for them.
It’s also essential to follow post-treatment instructions carefully, as this can help prevent complications and ensure a smooth recovery.
Making sure the filler material is used properly and avoiding excessive use or overfilling can also reduce the risk of complications.
Get Your Dermal Filler Consultation with Dr. Laura Geige at It’s Me and You Clinic
A reputable and licensed healthcare professional will be aware of potential risks and take necessary precautions to minimize them, such as using sterile equipment and following established protocols for injection and aftercare.
It’s crucial to find a qualified professional who has experience with tear trough fillers and is up-to-date on the latest techniques and materials.
The use of approved filler materials and adherence to proper injection and post-treatment guidelines can significantly reduce the risk of complications associated with tear trough fillers.
A thorough understanding of the potential risks and benefits, as well as careful monitoring during and after treatment, are essential for ensuring a safe and successful outcome.
Health Concerns for Certain Individuals
The use of Tear Trough Fillers has become increasingly popular in recent years, particularly among individuals seeking to reduce the appearance of dark circles and add volume under the eyes.
However, like any other medical treatment, there are certain health concerns that patients with underlying medical conditions should be aware of when considering this procedure.
For individuals with autism spectrum disorder, tear trough fillers may pose a risk due to their increased sensitivity and potential for allergic reactions to the filler materials used.
Patients with dysautonomia should exercise caution when considering tear trough fillers, as they may be more prone to complications such as numbness or tingling sensations in the facial area.
Individuals with a history of bleeding disorders, such as hemophilia, should consult their physician before undergoing a tear trough filler procedure, as the risk of excessive bleeding may be higher.
Patients with active infections or suspicion of infection in the facial area should wait until their condition has been fully treated and cleared by their healthcare provider before proceeding with the tear trough filler treatment.
Individuals with a weakened immune system, such as those with HIV/AIDS or undergoing chemotherapy, may be more susceptible to complications from tear trough fillers, including the spread of infection or an allergic reaction.
Those with a history of keloid scarring or previous facial trauma should discuss their individual risks with their physician before undergoing a tear trough filler treatment, as these conditions may increase the risk of complications such as granuloma formation.
Patients with allergies to local anesthetics or other medications used during the procedure may require alternative treatments or special precautions to be taken during the administration of the filler.
The use of botox-like toxins in tear trough fillers can also pose risks for individuals with migraines or seizures, as these conditions may be exacerbated by the toxin’s effects on nerve function.
Furthermore, patients with glaucoma should exercise caution when considering tear trough fillers, as the procedure may cause a temporary increase in intraocular pressure.
Individuals with a history of facial trauma or surgery, such as rhinoplasty or blepharoplasty, should consult their physician before undergoing a tear trough filler treatment to discuss potential risks and complications.
Lastly, patients with uncontrolled thyroid disease may require special precautions when undergoing a tear trough filler treatment, as the procedure can exacerbate symptoms such as bulging eyes or eyelid retraction.
To minimize risks, it is essential for individuals with underlying medical conditions to consult with their physician before undergoing a tear trough filler treatment and to carefully review the potential benefits and risks associated with this procedure.
Tear trough fillers are a popular cosmetic treatment used to address hollows under the eyes, creating a more youthful and rejuvenated appearance.
However, for individuals with bleeding disorders, tear trough filler injections may pose a higher risk of complications.
Bleeding disorders, such as hemophilia or von Willebrand disease, affect the body’s ability to form blood clots, making it harder for the body to stop bleeding when injured.
When tear trough fillers are injected into the skin, there is a risk of bleeding, bruising, and swelling at the injection site.
For individuals with bleeding disorders, this increased risk of bleeding can lead to more severe complications, such as:
Extended bleeding or bruising that may take longer than usual to resolve
Increased risk of hematoma formation, which is a collection of blood outside of blood vessels that can put pressure on surrounding tissues
Potential damage to adjacent facial structures, such as nerves and blood vessels, due to increased bleeding and swelling
Furthermore, individuals with bleeding disorders may be more susceptible to complications from the filler materials themselves.
Silicone-based fillers, for example, can cause an allergic reaction or granuloma formation in some individuals, which can lead to further inflammation and scarring
In rare cases, the body may react to the filler by forming a blood clot that can block the flow of blood to the face, leading to tissue necrosis (death) and permanent disfigurement.
It is essential for individuals with bleeding disorders to consult with their healthcare provider or a qualified healthcare professional before undergoing any cosmetic treatment, including tear trough fillers.
A thorough evaluation and discussion of potential risks and complications can help determine whether this treatment is safe and suitable for each individual.
Additionally, some clinics may offer special precautions or alternative treatments for individuals with bleeding disorders, such as:
Using platelet-rich plasma (PRP) therapy instead of fillers
Elevating the head while the filler is administered to reduce swelling and bruising
Frequent follow-up appointments to monitor for potential complications
In some cases, alternative treatments may be more suitable for individuals with bleeding disorders, such as blepharoplasty (eyelid surgery) or laser skin rejuvenation.
This section highlights the importance of carefully considering individual health needs when selecting a cosmetic treatment, such as _Tear Trough Fillers_. Patients with pre-existing autoimmune disorders, such as *_Lupus_* or *_Rheumatoid Arthritis_*, may be at increased risk for adverse reactions to certain treatments.
Autoimmune disorders occur when the body’s immune system mistakenly attacks its own tissues and organs. In the case of *_Lupus_* and *_Rheumatoid Arthritis_*, this can lead to inflammation, tissue damage, and potentially life-threatening complications.
_Tear Trough Fillers_, commonly used to treat _periorbital hooding_ (dark circles under the eyes) and _nasolabial folds_ (lines between the nose and mouth), typically contain *_hyaluronic acid_* or *_calcium hydroxylapatite_*. While generally considered safe, certain individuals with autoimmune disorders may be more susceptible to complications.
One of the primary concerns is the potential for *_immune complex formation_*, where the immune system mistakenly identifies the filler material as foreign and launches an immune response. This can lead to inflammation, scarring, or other adverse reactions.
Patients with *_Lupus_* are at increased risk due to the disease’s known association with *_neutrophil activation_*. Neutrophils are a type of white blood cell that play a key role in the body’s immune response. However, when activated, they can release pro-inflammatory chemicals that exacerbate inflammation and tissue damage.
Individuals with *_Rheumatoid Arthritis_* may also be more susceptible due to their compromised immune system. The disease is characterized by chronic inflammation, which can lead to joint damage and other systemic complications. While the filler material itself is not typically a direct cause of inflammation, patients with _Rheumatoid Arthritis_ may be more prone to developing inflammatory reactions.
Furthermore, patients with autoimmune disorders often require concurrent medications that can interact with *_Tear Trough Fillers_*. For example, certain immunosuppressants or corticosteroids may increase the risk of adverse reactions when combined with fillers.
In order to minimize risks, it is essential for individuals with autoimmune disorders to consult with their primary care physician and a board-certified dermatologist or plastic surgeon before undergoing treatment. A thorough medical evaluation will help determine whether _Tear Trough Fillers_ are safe and suitable for each individual’s specific needs.
A comprehensive pre-treatment assessment should include a review of the patient’s medical history, current medications, and any allergies or sensitivities. The doctor should also perform a physical examination and potentially order additional tests to assess overall health and potential risks.
Once cleared for treatment, patients with autoimmune disorders should take steps to mitigate their risk, such as:
- Following post-treatment instructions carefully
- Avoiding strenuous exercise or activities that may exacerbate inflammation
- Monitoring for signs of infection or allergic reaction
- Maintaining a healthy lifestyle, including a balanced diet and regular exercise
By taking these precautions and working closely with their healthcare team, individuals with autoimmune disorders can minimize their risk and safely undergo _Tear Trough Filler_ treatment.
Dermal fillers have become increasingly popular in recent years for various cosmetic purposes, including reducing the appearance of fine lines, wrinkles, and age-related facial sagging.
However, while generally considered safe when used properly, dermal fillers can pose serious health risks to certain individuals with pre-existing medical conditions.
The FDA has issued warnings about the potential dangers of using dermal fillers for patients with bleeding disorders.
These individuals are at a higher risk of experiencing serious complications, including but not limited to, excessive bruising, swelling, and blood vessel rupture.
Bleeding disorders can range from mild to severe and may include conditions such as hemophilia or von Willebrand disease.
Patients with these conditions should consult with their doctor before undergoing any cosmetic procedure that involves dermal fillers.
In addition to bleeding disorders, there are other health concerns that individuals should be aware of when considering the use of dermal fillers.
Patients with a history of allergies to certain ingredients in dermal fillers, such as lidocaine or aluminum, may experience an allergic reaction upon injection.
Individuals with autoimmune disorders, such as rheumatoid arthritis or lupus, may be more susceptible to adverse reactions from dermal fillers.
Additionally, patients with a history of stroke or transient ischemic attack (TIA) should exercise caution when using dermal fillers, as these conditions can increase the risk of bleeding and other complications.
Furthermore, individuals with certain eye conditions, such as dry eye syndrome or blepharitis, may be at higher risk for complications from dermal fillers used in the tear trough area.
Other health concerns that patients should be aware of when considering dermal fillers include a history of keloid scarring, poor wound healing, and previous adverse reactions to dermal fillers.
Patients with these conditions or who have experienced complications from previous dermal filler use should discuss their individual risks and benefits with a qualified healthcare professional before undergoing treatment.
Regulation and Safety Monitoring
The world of **dermal fillers** has experienced tremendous growth in recent years, with various treatments available to address concerns such as fine lines, wrinkles, and age-related changes in facial appearance. However, amidst this growing demand, the importance of ensuring the safety and efficacy of these products cannot be overstated.
Regulatory agencies play a crucial role in overseeing the development, approval, and monitoring of **tear trough filler** ingredients to ensure they are safe for human use. These agencies work tirelessly to balance the need for innovation with the need for strict adherence to safety standards.
The primary regulatory agency responsible for overseeing the safety of cosmetic products, including fillers, is the U.S. Food and Drug Administration (FDA). The FDA’s Center for Biologics Evaluation and Research (CBER) is specifically responsible for approving **biologic dermal fillers**, such as those used to treat tear troughs.
The regulatory process for new cosmetic ingredients, including fillers, involves several stages:
- Preclinical testing: The manufacturer conducts laboratory tests to assess the safety and efficacy of the filler in vitro (in a lab dish) and in vivo (in animal models).
- Clinical trials: Human clinical trials are conducted to gather more extensive data on the filler’s safety and effectiveness. These trials must meet specific regulatory requirements, including blinded, placebo-controlled designs.
- Submission for FDA approval: The manufacturer submits a New Drug Application (NDA) or Abbreviated New Drug Application (ANDA) to the FDA, providing detailed information on the filler’s composition, manufacturing process, and clinical trial data.
- Post-marketing surveillance: After the filler is approved for marketing, it undergoes ongoing monitoring through post-market studies and adverse event reporting. Regulatory agencies review these data to ensure the product remains safe and effective over time.
The FDA also requires manufacturers of tear trough fillers to conduct post-approval studies**_, which investigate the long-term safety and efficacy of the product in a broader population.
Another critical aspect of ensuring safety is **compliance with Good Manufacturing Practice (GMP)** standards. Manufacturers must adhere to GMP guidelines to ensure their production processes meet minimum safety and quality standards.
Labeling and instructions are also crucial in ensuring patient safety. Regulatory agencies require manufacturers to provide clear, concise labeling that outlines the benefits and risks associated with each filler product.
In addition to FDA oversight, many countries have their own regulatory agencies responsible for reviewing the safety of cosmetic products, including fillers.
The consequences of inadequate regulation can be severe. **Adverse events** related to tear trough filler use, such as inflammation, allergic reactions, or even more serious complications like **facial paralysis**, can result from the use of non-compliant products or inadequate labeling.
Given the potential risks associated with certain fillers, it is essential for patients to undergo thorough consultation and screening before undergoing treatment. This may include a detailed medical history, discussion of their expectations and concerns, and selection of a qualified healthcare professional or dermatologist for administration of the filler.
The regulation and safety monitoring of cosmetic procedures like tear trough filler injections are crucial to ensuring the well-being of patients.
The US Food and Drug Administration (FDA) plays a pivotal role in regulating these types of medical treatments, including the use of fillers such as hyaluronic acid, calcium hydroxylapatite, and poly-L-lactic acid.
Tear trough filler injections are a type of cosmetic procedure that involves injecting fillers into the tear trough area to reduce the appearance of dark circles and fine lines under the eyes.
However, like any medical treatment, there are potential risks and complications associated with this procedure, including allergic reactions, infection, and necrotizing fasciitis.
The FDA has established guidelines for the safe use of fillers in cosmetic procedures, including requirements for the preparation, handling, and administration of these substances.
Regulatory bodies such as the FDA and the American Society for Dermatologic Surgery (ASDS) also provide guidance on the proper training and qualifications of practitioners performing tear trough filler injections.
The importance of informed consent cannot be overstated in this context. Patients must be fully aware of the potential benefits and risks associated with the procedure, including the possible need for repeated treatments or additional procedures.
Safety monitoring is also a critical component of regulation in this area. This includes tracking reports of adverse events and complications, as well as conducting post-marketing surveillance to identify any long-term effects of fillers.
The use of Quality Control (QC) measures, such as testing for purity and potency, is also essential in ensuring the safety of fillers used in cosmetic procedures like tear trough filler injections.
In addition, regulatory bodies often require practitioners to participate in continuing education and training programs to stay up-to-date on the latest techniques and safety protocols.
The potential consequences of not adhering to these regulations can be severe. Complications from tear trough filler injections can result in long-term scarring, disfigurement, or even permanent damage.
Therefore, it is essential for practitioners to follow established guidelines and protocols when performing cosmetic procedures like tear trough filler injections, and for patients to choose qualified and experienced practitioners who adhere to the highest standards of safety and regulation.
The use of sterile equipment, single-use products, and immediate post-procedure follow-up care can also help minimize the risk of complications and ensure a safe and successful outcome for patients.
In conclusion, regulation and safety monitoring are critical components of ensuring the safe use of fillers in cosmetic procedures like tear trough filler injections. By following established guidelines and protocols, practitioners can help minimize the risk of complications and provide patients with optimal results.
The regulation and safety monitoring of injectable fillers, including those used for tear troughs, are crucial to ensure their safe use and minimize the risk of adverse effects.
According to a study published by the American Academy of Otolaryngology Head and Neck Surgery Foundation, the US Food and Drug Administration (FDA) closely monitors dermal fillers for safety and efficacy. This monitoring involves reviewing clinical trial data, gathering post-marketing reports, and conducting inspections of manufacturing facilities.
The FDA has a tiered regulatory approach to injectable fillers, which includes:
- Class I: These products have a history of safe use in the US and do not require FDA approval. Examples include hyaluronate and glycerin-based fillers.
- Class II: These products have lower risk but may cause significant side effects if used improperly. They require pre-market clearance from the FDA, which involves review of clinical trial data to ensure efficacy and safety.
- Class III: These products are considered high-risk and require post-marketing surveillance to gather more information about their effectiveness and safety. Examples include autologous fat grafting and some advanced biologic fillers.
Regulatory agencies, such as the FDA, work with professional societies and manufacturers to ensure that injectable fillers are safe and effective for treatment of various conditions, including tear troughs.
Tear trough fillers, also known as malar fillers, are used to reduce the appearance of fine lines, wrinkles, and fat loss under the eyes. Common fillers used for this purpose include:
- Calcium hydroxylapatite (Radiesse)
- Hyaluronic acid (Restylane, Juvederm)
- Polilactic acid (Sculptra)
While tear trough fillers are generally considered safe when used by experienced healthcare professionals, potential complications can occur. These may include:
- Edema and bruising
- Infection
- Nerve damage or numbness
- Asymmetry or unevenness
A thorough evaluation and informed consent are essential before undergoing tear trough filler treatment. Patients should discuss the potential risks, benefits, and alternatives to fillers with their healthcare provider.
In addition to FDA monitoring, professional organizations, such as the American Society of Plastic Surgeons (ASPS) and the American Academy of Otolaryngology Head and Neck Surgery Foundation, provide guidelines for safe use and treatment planning of injectable fillers, including tear troughs.
Regulation and safety monitoring are crucial aspects of any medical procedure, including *_tear trough filler_* treatment.
The use of *_tear trough fillers_*, such as hyaluronic acid or calcium hydroxylapatite, has become increasingly popular in recent years due to their ability to effectively address the visible signs of aging under the eyes.
However, like any medical procedure, *_tear trough filler_* treatment carries certain risks and complications, which can be minimized by choosing a qualified practitioner with extensive experience in administering these products.
Schedule a Dermal Filler Appointment with Dr. Laura Geige Now
As per guidelines set by the American Society of Plastic Surgeons, patients should carefully select a board-certified dermatologist or plastic surgeon who has undergone specialized training in *_tear trough filler_* administration to ensure optimal results and minimize potential complications.
Qualification is key when it comes to *_tear trough filler_* treatment, as it reduces the risk of *_infection_*, *_allergic reactions_*, *_vascular occlusions_*, and other adverse events.
In addition to qualification, patients should also look for practitioners who have a proven track record of success with *_tear trough filler_* treatments, as this can be an indicator of their expertise and experience in administering these products.
The American Society of Plastic Surgeons also emphasizes the importance of following proper *_pre-procedure_* and *_post-procedure_* care instructions to ensure optimal results and minimize the risk of complications.
During the *_pre-procedure_* phase, patients should be thoroughly evaluated by their practitioner to identify any potential risks or contraindications, such as bleeding disorders or previous *_tear trough filler_* reactions.
In the *_post-procedure_* phase, patients should carefully follow their practitioner’s instructions regarding rest, activity level, and symptom management to minimize the risk of complications and promote optimal healing.
Furthermore, patients should also be aware of the potential risks associated with *_tear trough filler_* treatment, such as *_swelling_*, *_bruising_*, *_redness_*, *_eye irritation_*, and *_visual disturbances_*.
In some cases, these complications can be severe and require immediate medical attention.
Therefore, it is essential for patients to carefully weigh the potential benefits of *_tear trough filler_* treatment against the potential risks and to choose a qualified practitioner who has extensive experience in administering these products.
The American Society of Plastic Surgeons also recommends that patients undergo a thorough consultation with their practitioner before undergoing *_tear trough filler_* treatment to discuss their individual concerns, risks, and benefits.
During this consultation, the patient should ask questions regarding the practitioner’s qualifications, experience, and success rates with *_tear trough filler_* treatments, as well as any potential risks or complications associated with these products.
In addition, patients should also be aware of the available options for *_tear trough filler_* treatment, such as different types of fillers, injection techniques, and post-procedure care protocols.
This knowledge will enable patients to make informed decisions about their *_tear trough filler_* treatment and ensure that they receive optimal results while minimizing potential risks.
Read more about Line the Studio here. Read more about Critic Forever here. Read more about K’s P Rules Cakes here. Read more about Josie Barrett here.
